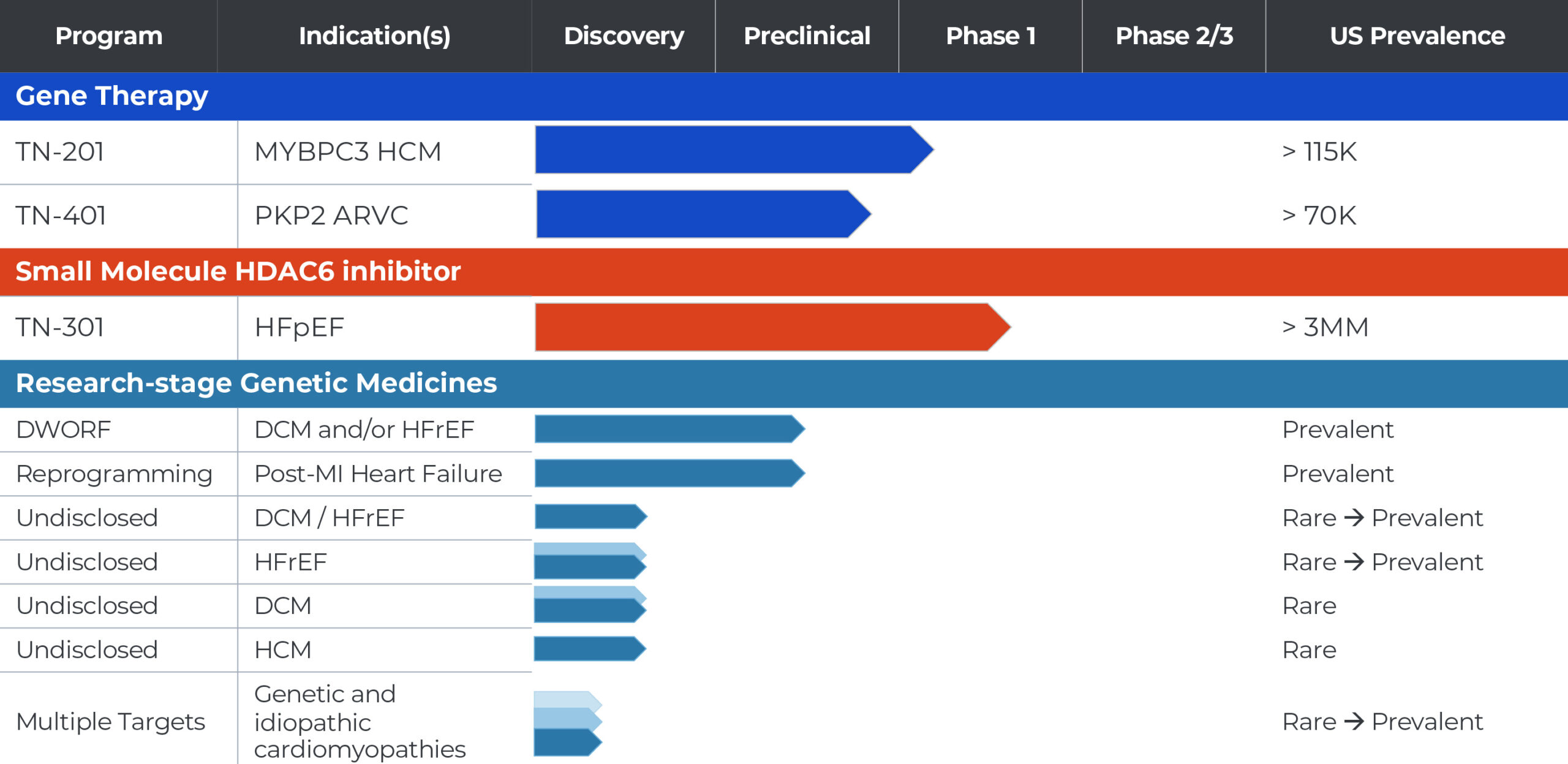
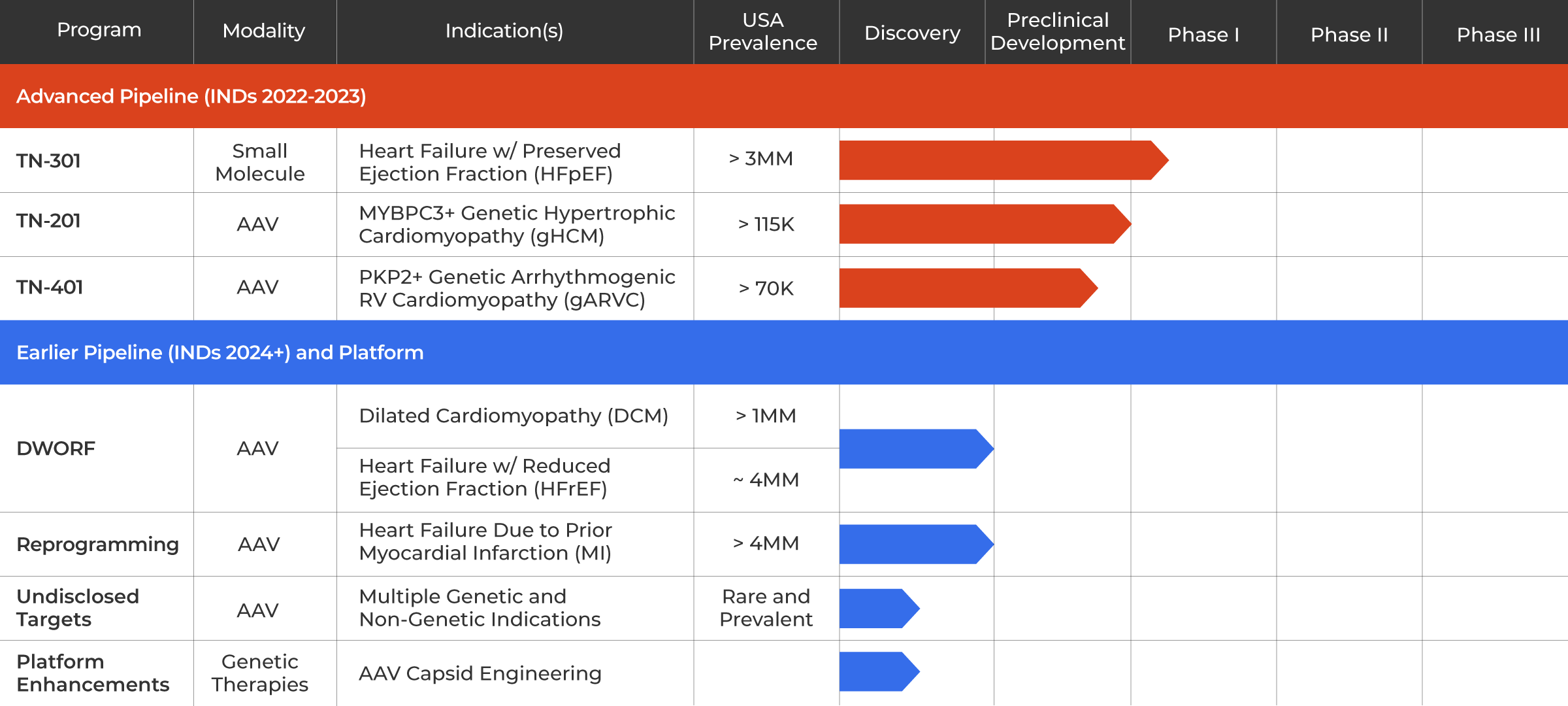
TN-201: MYBPC3 Gene Therapy Program for Genetic HCM
We are developing TN-201, an adeno-associated virus (AAV)-based gene therapy designed to treat adults and children with HCM due to MYBPC3 gene mutations, the most prevalent form of genetic HCM. MYBPC3-associated HCM is a chronic, progressive condition estimated to affect more than 115,000 patients in the United States. MYBPC3 gene mutations can cause the heart walls to become significantly thickened, potentially leading to serious complications including fibrosis, abnormal heart rhythms, cardiac dysfunction, heart failure and sudden cardiac death in some adults and children.
TN-201 is intended to address the underlying cause of disease by delivering a fully functional MYBPC3 gene to restore normal levels of MYBPC3 protein with the hope of potentially halting disease progression and reversing the course of genetic HCM after a single treatment. In preclinical studies (presented at the ASGCT Annual Meeting and ESGCT Congress in 2021), we have demonstrated significant and durable disease reversal and survival benefit after a single dose, as well as tolerability in mice and non-human primates (NHPs).
In early 2023, we received clearance of our Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) to begin clinical testing of TN-201. We are initiating a Phase 1b clinical trial enrolling symptomatic adults who have been diagnosed with MYBPC3-associated nonobstructive HCM and expect to begin patient dosing in the third quarter of 2023. The Phase 1b clinical trial is designed to assess the safety, tolerability and clinical efficacy of a one-time intravenous dose of TN-201.
MYBPC3 Program Publications & Presentations
TN-401: PKP2 Program for Genetic ARVC
We are developing TN-401, an adeno-associated virus (AAV)-based gene therapy designed to deliver a functional PKP2 gene in adults with ARVC due to PKP2 gene mutation. PKP2-associated ARVC is estimated to affect more than 70,000 patients in the United States. These mutations can cause enlargement of the right ventricle in affected individuals and the gradual replacement of heart muscle with fibrotic tissue and fatty deposits. ARVC is characterized by severely abnormal heart rhythms (arrhythmia) that can make it difficult for the heart to function properly and result in sudden cardiac death in some adults and children.
In preclinical studies (presented at the ASGCT Annual Meeting and HRS Heart Rhythm event in 2022) of PKP2 knockout mice, we have demonstrated prevention and reversal of disease progression and survival benefit following a single dose for gene therapy. We believe these data are among the first demonstration of durable disease modification, survival benefit and prevention of arrhythmia using an AAV:PKP2 gene therapy.
In 2022, TN-401 received an Orphan Drug Designation from the FDA. We are currently conducting IND enabling studies for the program and expect to submit an IND in 2023 to allow us to conduct a clinical trial of TN-401 in patients with ARVC caused by PKP2 mutations.
DWORF Gene Therapy Program for Dilated Cardiomyopathy (DCM)
We are developing an AAV-based gene therapy designed to deliver the DWORF gene for patients with DCM, estimated to affect about one million patients in the United States. DCM is a progressive and life-threatening disease that causes left ventricle (LV) enlargement, LV wall thinning, insufficient contraction, reduced blood flow, ventricular arrhythmias, and can result in premature morbidity and need for heart transplant in affected individuals. DWORF is a muscle specific micro-peptide first discovered by our co-founder Eric Olson, Ph.D. that acts on the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) pathway, widely considered to be a promising target in HF.
We and our academic collaborators have accumulated significant preclinical in vivo proof-of-concept evidence for the therapeutic benefit of overexpression of the DWORF gene in multiple murine models, including models of gDCM and HFrEF, as well as tolerability in murine models. Based on publicly available information to date, we believe these are the first demonstrations of the potential benefit of AAV:DWORF.
This program is currently at the candidate selection stage.
DWORF Gene Therapy Program Publications & Presentations
TN-301: HDAC6 Inhibitor for HFpEF
We are developing TN-301, a first-in-class small molecule histone deacetylases (HDAC) 6 inhibitor for the potential treatment of heart failure with preserved ejection fraction (HFpEF). HFpEF is estimated to affect more than three million people in the U.S. alone, and its prevalence is growing. Risk factors for HFpEF include hypertension, kidney disease, inflammation associated with aging and metabolic disorders such as diabetes and obesity. HFpEF is characterized by a stiffening of the heart muscle resulting in an inability for the left ventricle to relax properly during normal heart rhythm, referred to as diastolic dysfunction. Symptoms initially include fatigue, shortness of breath and tissue swelling, resulting in reduced capacity for physical activity. Over time, this results in a substantial limitation in activities impacting quality of life. HFpEF patients are at risk of hospitalizations due to heart failure symptoms and premature death.
There are several cellular processes thought to underly the pathophysiology of HFpEF, including increases in fibrosis and inflammation as well as defects in metabolism. TN-301 was discovered using our proprietary Precision Medicine platform approach. TN-301 is a highly selective inhibitor of HDAC6, an enzyme in the cell cytoplasm that interacts with proteins, including tubulin, to coordinate cellular processes known to directly contribute to HFpEF. In preclinical studies, Tenaya’s HDAC6 inhibitors have been shown to increase cardiac function, reduce inflammation and fibrosis, reverse diastolic dysfunction and improve glucose tolerance, as well as achieve comparable in vivo efficacy to the SGLT2 inhibitor empagliflozin in a model of HFpEF.
A Phase 1 clinical study to evaluate the safety and tolerability of TN-301 is current underway. Tenaya anticipates sharing the first clinical data from this trial in the second half of 2023.
HDAC6 Inhibitor Publications & Presentations
Reprogramming Program for Heart Failure Due to Prior Myocardial Infarction (MI)
We are developing an AAV-based approach to cellular regeneration that involves converting (or reprogramming) existing cardiac fibroblasts (CFs) within the heart to turn into new cardiomyocytes (CMs) and to replace cells permanently lost due to MI. There are estimated to be more than four million patients in the United States living with HF due to prior MI. The loss of CMs in affected individuals permanently impairs heart contraction, leading to HF and potentially fatal arrhythmias, and the death of approximately 5% to 10% of MI survivors within the first year. There are currently no approved treatments that address the underlying loss of heart tissue.
The potential utility of our unique approach to creating new CMs was first demonstrated by our co-founder Deepak Srivastava, M.D. We have discovered a proprietary combination of three genes that can drive robust in vivo reprogramming of CFs to CMs when delivered together in a single AAV capsid. We have demonstrated significant and durable disease reversal as well as tolerability in multiple small and large animal models. Based on publicly available information to date, we believe our results in a pig model of HF due to prior MI represent the first-ever successful demonstration of the potential beneficial effect of this approach in a human-sized heart.
This program is currently at candidate selection stage.
