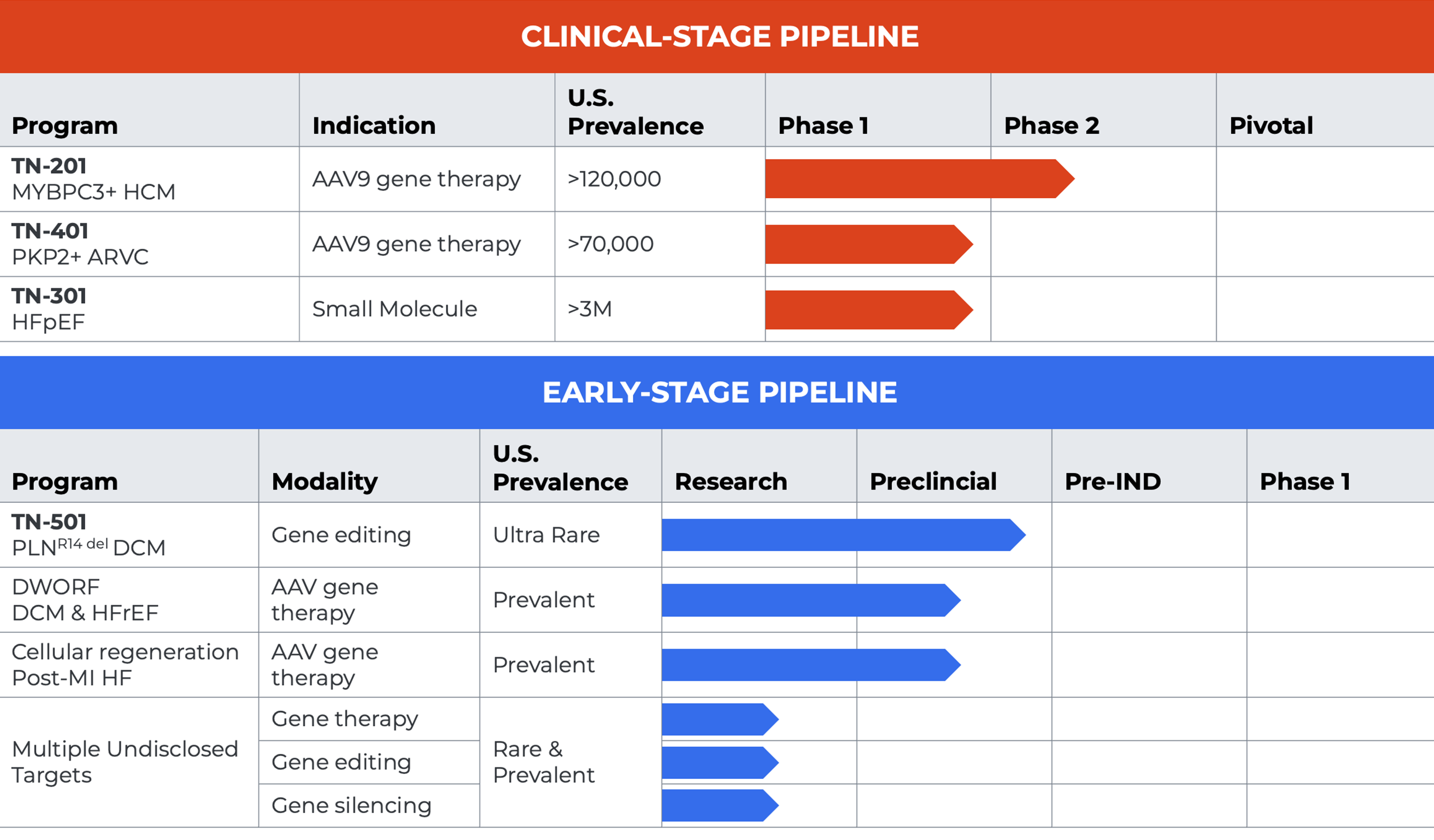
Tenaya’s Clinical-stage Programs
TN-201: MYBPC3 Gene Therapy Program for Genetic HCM
We are developing TN-201, an adeno-associated virus (AAV)-based gene therapy for the treatment of MYBPC3-associated HCM. Variants in the Myosin Binding Protein C3 (MYBPC3) gene are the most common genetic cause of hypertrophic cardiomyopathy (HCM), a chronic progressive condition affecting an estimated 120,000 adults, teens, children and infants in the U.S. alone. Mutations of the MYBPC3 gene result in insufficient levels of the MyBP-C protein, which is critical in modulating the contraction of the heart. In MYBPC3-associated HCM the heart becomes hypercontractile and the left ventricle thickens, resulting in symptoms such as chest pain, shortness of breath, palpitations and fainting that interfere in activities of daily living. Patients whose disease is caused by MYBPC3 mutations are more likely than those with non-genetic forms of HCM to experience earlier disease onset and have high rates of serious outcomes, including heart failure symptoms, abnormal heart rhythms, stroke and sudden cardiac arrest or death.
TN-201 is intended to address the underlying cause of disease by delivering a fully functional MYBPC3 gene to heart muscle cells in order to restore normal levels of MyBP-C protein with the hope of potentially halting disease progression and reversing the course of genetic HCM after a single treatment. We are conducting the MyPEAKTM-1 Phase 1b/2 clinical trial (Clinicaltrials.gov ID: NCT05836259), an ongoing, multi-center, open-label, dose-escalating study designed to assess the safety, tolerability and clinical efficacy of a one-time intravenous infusion of TN-201 gene replacement therapy. MyPEAK-1 is testing doses of 3E13 vg/kg and 6E13 vg/kg in two cohorts of three patients. Initial data from the first three patients to receive TN-201 were presented at the 2025 American College of Cardiology (ACC) Annual Meeting.
The U.S. Food and Drug Administration has granted TN-201 Fast Track, Orphan Drug and Rare Pediatric Drug Designations. TN-201 has also received orphan medicinal product designation from the European Commission.
TN-401: PKP2 Program for Genetic ARVC
We are developing TN-401, a gene therapy designed to deliver a functional Plakophilin-2 (PKP2) gene to adults with arrhythmogenic right ventricular cardiomyopathy (ARVC) due to PKP2 gene mutation. Mutations in the PKP2 gene are the most common genetic cause of ARVC, with more than 40% of ARVC patients carrying pathogenic variants, resulting in an estimated 70,000 people affected in the United States alone. PKP2 mutations result in insufficient expression of a protein needed for the proper functioning of desmosome, a complex that maintains physical connections and electrical signaling between heart muscle cells. As the desmosome structure is impaired, cardiac muscle cells are progressively replaced by fibrofatty tissue and electrical pulses in the heart become unstable, resulting in adverse remodeling and irregular heart rhythms. ARVC is characterized by severely abnormal heart rhythms (arrhythmia) that can make it difficult for the heart to function properly and result in sudden cardiac death in some adults and children.
TN-401 is designed to deliver a working PKP2 gene into heart muscle cells using an AAV9 capsid where the functional PKP2 gene produces the missing protein, restoring function and reversing or slowing progression of disease by addressing the genetic mutation most frequently underlying ARVC. We are conducting the RIDGETM-1 Phase 1b clinical trial (ClinicalTrials.gov ID: NCT06228924) of TN-401 in patients with PKP2-associated ARVC. The RIDGE-1 Phase 1b clinical trial is a multi-center, open-label, dose escalation study being conducted in the U.S. and UK. RIDGE-1 will assess the safety, tolerability and preliminary clinical efficacy of a one-time intravenous infusion of TN-401. RIDGE-1 is designed to test doses of 3E13vg/kg and 6E13 vg/kg. The first cohort of three patients have been enrolled in RIDGE-1 and initial data is expected to be reported in the fourth quarter of 2025.
TN-401 received an Orphan Drug and Fast Track Designations from the FDA. TN-401 has also received orphan medicinal product designation from the European Commission.
TN-301: HDAC6 Inhibitor for HFpEF
TN-301 is a first-in-class small molecule histone deacetylases (HDAC) 6 inhibitor for the potential treatment of heart failure with preserved ejection fraction (HFpEF) and related cardio/muscular disease. TN-301 was discovered using our proprietary Precision Medicine platform approach. TN-301 is a highly selective inhibitor of HDAC6, an enzyme in the cell cytoplasm that interacts with proteins, including tubulin, to coordinate cellular processes known to directly contribute to HFpEF and other diseases. Based on TN-301’s multi-modal mechanism of action, TN-301 may have future potential in a number of cardiometabolic conditions. In preclinical studies, Tenaya’s HDAC6 inhibitors have been shown to increase cardiac function, reduce inflammation and fibrosis, reverse diastolic dysfunction and improve glucose tolerance, as well as achieve comparable in vivo efficacy to the SGLT2 inhibitor empagliflozin in a model of HFpEF. In a Phase 1 clinical study of healthy participants, TN-301 demonstrated safety and tolerability in healthy participants with dose-proportional pharmacokinetics and robust target engagement. Taken together, these data support continued development of TN-301 as a potential treatment for patients with HFpEF and other severe diseases – including those outside of cardiology- in which inflammation, fibrosis and metabolic dysregulation may be implicated. We believe that TN-301’s late-stage development and commercialization would best be led by a strategic partner with the global resources to explore the full potential of the molecule.
Preclinical Programs
While we are firmly focused on advancing our lead product candidates through the clinic, we have multiple early-stage programs progressing through preclinical development using various therapeutic approaches, including gene addition, gene editing, gene silencing, and cellular regeneration to address other forms of rare and/or prevalent heart disease.