
TN-401
Our clinical-stage gene therapy for PKP2-associated ARVC
Mutations in plakophilin-2 are the most common genetic cause of ARVC, resulting insufficient levels of critical desmosomal proteins needed to maintain the structural integrity and cell-to-cell signaling of heart muscle cells. TN-401 gene replacement therapy is designed address the underlying cause of disease by delivering a working PKP2 gene to the heart.
ARVC and Genetics
- Epidemiology of the inherited cardiomyopathies
Nature Reviews Cardiology, Sept 7, 2020
- Arrhythmogenic Right Ventricular Cardiomyopathy Prevalence and Arrhythmic Outcomes in At-Risk Family Members: A Systematic Review and Meta-Analysis
Circulation: Genomic and Precision Medicine, May 17, 2022 - Population Prevalence of Premature Truncating Variants in Plakophilin-2 and Association With Arrhythmogenic Right Ventricular Cardiomyopathy: A UK Biobank Analysis
Circulation: Genomic and Precision Medicine, May 10, 2022 - Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital
International Journal of Cardiology, Dec 2004
- International Evidence Based Reappraisal of Genes Associated With Arrhythmogenic Right Ventricular Cardiomyopathy Using the Clinical Genome Resource Framework
Circulation: Genomic and Precision Medicine, Apr 8, 2021 - State of the Art Review on Genetics and Precision Medicine in Arrhythmogenic Cardiomyopathy
International Journal of Molecular Sciences, Sept 10, 2020 - Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers
European Heart Journal, Jan 23, 2015 - Molecular Insights into Arrhythmogenic Right Ventricular Cardiomyopathy Caused by Plakophilin-2 Missense Mutations
Circulation: Genomic and Precision Medicine, Jul 9, 2012 - Penetrance of Mutations in Plakophilin-2 Among Families With Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy
Journal of the American College of Cardiology, Oct 3, 2006
- High penetrance and similar disease progression in probands and in family members with arrhythmogenic cardiomyopathy
European Heart Journal, Sept 1, 2019 - Management of patients with Arrhythmogenic Right Ventricular Cardiomyopathy in the Nordic countries
Scandinavian Cardiovascular Journal, Sept 23, 2015 - Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members
Circulation: Genomic and Precision Medicine, Mar 27, 2015 - Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: Proposed Modification of the Task Force Criteria
Circulation, Feb 19, 2010
- Natural Course of Electrocardiographic Features in Arrhythmogenic Right Ventricular Cardiomyopathy and Their Relation to Ventricular Arrhythmic Events
Journal of the American Heart Association, Aug 19, 2024 - Arrhythmic risk stratification in arrhythmogenic right ventricular cardiomyopathy
EP Europace, Nov 3, 2023
- Genotype–phenotype correlation in arrhythmogenic right ventricular cardiomyopathy—risk of arrhythmias and heart failure
Journal of Medical Genetics, Nov 15, 2022 - Longitudinal Prediction of Ventricular Arrhythmic Risk in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy
Circulation: Arrhythmia and Electrophysiology, Oct 31, 2022 - Arrhythmic risk prediction in arrhythmogenic right ventricular cardiomyopathy: external validation of the arrhythmogenic right ventricular cardiomyopathy risk calculator
European Heart Journal, Jun 29, 2022 - Arrhythmogenic Right Ventricular Cardiomyopathy
JACC, Apr 18, 2022
- Incidence and predictors of sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy: a pooled analysis
EP Europace, Mar 17, 2022 - Association of Premature Ventricular Contraction Burden on Serial Holter Monitoring With Arrhythmic Risk in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy
JAMA Cardiology, Febr 23, 2022 - Sudden Cardiac Death Prediction in Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC): A Multinational Collaboration
Circulation: Arrhythmia and Electrophysiology, Dec 9, 2020 - 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: Executive summary
Heart Rhythm Society, Nov 2019 - Primary Prevention of Sudden Cardiac Death With Implantable Cardioverter-Defibrillator Therapy in Patients With Arrhythmogenic Right Ventricular Cardiomyopathy
The American Journal of Cardiology, Apr 1, 2019 - A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy
European Heart Journal, Mar 27, 2019 - Incidence and Predictors of Implantable Cardioverter-Defibrillator Therapy in Patients With Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Undergoing Implantable Cardioverter-Defibrillator Implantation for Primary Prevention
Journal of the American College of Cardiology, Sept 27, 2011
- Complications of implantable cardioverter- defibrillator treatment in arrhythmogenic right ventricular cardiomyopathy
EP Europace, Jul 19, 2021 - Treatment of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia
Circulation, Jul 27, 2015
How TN-401 Works
- AAV9:PKP2 improves heart function and survival in a Pkp2-deficient mouse model of arrhythmogenic right ventricular cardiomyopathy
Tenaya Publication, Mar 18, 2024 - PKP2 Gene Therapy Reduces Ventricular Arrhythmias, Reverses Ventricular Remodeling, Improves Heart Function, and Reduces Mortality in a Mouse Model of Arrhythmogenic Right Ventricular Cardiomyopathy
HRS Heart Rhythm 2022, May 2, 2022
- AAV9 Exhibits Superior Cardiomyocyte Transgene Expression in vivo in Murine and Non-Human Primate Models Relative to AAVrh.10 and AAVrh.74 and Mediates Greater Efficacy in a Cardiomyopathy Disease Model
2024 American Society of Gene & Cell Therapy 27th Annual Meeting, May 8, 2024
RIDGETM-1 Phase 1b Clinical Trial
- Clinical trial brochure
- Open-label, Dose Escalation Study of Safety and Preliminary Efficacy of TN-401 in Adults With PKP2 Mutation-associated ARVC (RIDGE-1)
Tenaya Study | ClinicalTrials.gov Identifier: NCT06228924
Tenaya’s Non-interventional Study for PKP2-Associated ARVC
- RIDGE-1 Antibody Testing and Observational Study
- Non-interventional Study of Seroprevalence of Pre-existing Antibodies Against Adenovirus-associated Virus Vector (AAV9) and the Progression of Disease in Patients with Plakophilin 2 (PKP2)-associated Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
Tenaya Study | ClinicalTrials.gov identifier: NCT06311708 - Determining Eligibility for RIDGE-1, a Phase 1b Interventional Study to Evaluate Safety and Efficacy of TN-401 Gene Therapy in Adults with Plakophilin-2 (PKP2)-Associated Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC): Interim Results of an Observational Study to Assess Seroprevalence to Adeno-Associated Virus Serotype 9 (AAV9)
Calkins, H | Heart Rhythm Society Heart Rhythm 2025 | Apr 24, 2025 - Assessing Seroprevalence to Adeno-Associated Virus Serotype 9 in Preparation for RIDGE™-1, a Phase 1b First-in-Human Study to Evaluate Safety and Efficacy of TN-401 Investigational Gene Therapy in Adults with PKP2-Associated Arrhythmogenic Right Ventricular Cardiomyopathy
Calkins, H | 49th International Congress on Electrocardiology (ICE) | Jun 1, 2024
Frequently asked questions
Understanding AAV-based Gene Therapy
How many patients have been treated with AAV9-based gene therapies around the world?
The AAV9 serotype has been extensively studied in gene therapy, particularly for its ability to target tissues like heart, central nervous system (CNS), liver, and skeletal muscles. While it is difficult to provide an exact number of patients who have been treated with AAV9-based gene therapies across the world, either in clinical trials or with the approved gene therapy products, more than 5500 patients have been dosed in 50 countries with Zolgensma alone based on the latest figures released from Novartis.
What is the difference between gene therapy (replacement) and gene editing?
Gene replacement therapy and gene editing use genetic material to treat or prevent disease. Most gene therapy approaches work by delivering a functional gene into a cell to replace the genetic variant underlying a given condition and restore healthy protein levels necessary for proper function. Gene editing has a similar goal but differs by delivering genetic material that can directly edit pieces of DNA within a cell. This changes the instructions encoded by the DNA to correct the protein produced by the DNA and restore proper cell function. CRISPR-Cas9 is a common gene editing treatment approach for gene editing. (www.asgct.org)
How does AAV gene therapy differ from COVID vaccines?
AAV gene therapy is typically used for the treatment of genetic disorders. COVID vaccines are designed to protect against SARS-CoV-2 infection and COVID-19 disease in the general population (Hakroush & Tampe, 2021).
AAV gene therapy and COVID vaccines differ in their mechanisms of action and targets. AAV gene therapy involves the use of adeno-associated viruses (AAVs) to deliver therapeutic genes into cells, aiming to correct genetic disorders and uses a DNA-based approach (Speer et al., 2022). The COVID vaccine payload doesn’t enter the cell’s nucleus; instead, it directs the cells to produce spike protein at the ribosome in the cytoplasm, and is designed to stimulate an immune response against the SARS-CoV-2 virus, which causes COVID-19 (Hakroush & Tampe, 2021).
AAV gene therapy focuses on specific genetic mutations that cause diseases, aiming to provide a long-term or permanent correction at the genetic level (Speer et al., 2022). In contrast, COVID vaccines target the spike protein of the SARS-CoV-2 virus, which is responsible for viral entry into human cells and prompts an immune response without causing disease. (Hakroush & Tampe, 2021).
How does gene therapy produce the missing protein?
The AAV capsid is recognized by receptors at the cell surface of the host and taken into the cell via endosomes. Following endosomal escape, AAV enters the nucleus where it undergoes capsid uncoating to release the replacement gene, or “transgene”. The transgene undergoes circularization creating an episomal DNA, leading to gene expression that persists in postmitotic cells. Importantly, the episomal DNA does not integrate into the host DNA. (Wang D et al., 2019.) In the context of gene therapy, episomal DNA is used to introduce therapeutic genes into human cells. This approach can provide a safer alternative to integrating DNA into the host genome, which can lead to insertional mutagenesis. Episomal DNA refers to extrachromosomal genetic material that exists independently of chromosomal DNA within a cell. It is typically found in the form of circular or linear DNA molecules. It can replicate independently of the host cell’s chromosomal DNA, allowing for the maintenance and expression of genes encoded within it.
The episomal DNA now undergoes transcription to messenger RNA, which travels to the ribosomes for production of intended protein (e.g. TN-401 will restore functional Plakophilin 2 (PKP2) protein). Because the episome is stable and adult cardiomyocytes do not replicate, a single dose of TN-401 has the potential to provide a long-term durable response.
Gene Therapy Safety
What immunosuppression regimens are being used for AAV gene therapies?
Immunosuppression is required in AAV-mediated gene therapy clinical trials to maximize safety and efficacy for treated individuals. The immune system may recognize AAV vectors as foreign invaders and mount an immune response against them. Immunosuppression helps reduce this immune reaction, allowing the therapy to be more effective.
In Tenaya’s clinical trials of AAV9-based gene therapies, we utilize two components of the immunosuppressive regimen: Sirolimus and Prednisone. These are administered pre-dose on Day -7. Patients will be given a loading dose of sirolimus and then continue daily dosing based on sirolimus trough levels. Sirolimus will be continued for approximately 15 weeks, or until 1 week after the corticosteroids have been tapered off, or until the patient’s biomarkers and LFTs indicate that it can be stopped, whichever is longest. Following the administration of gene therapy, patients’ liver enzymes are closely monitored. Close monitoring continues throughout the tapering process. The duration and intensity of immunosuppression depend on the specific gene therapy being used and the patient’s immune status, requiring a balance between reducing immune rejection and minimizing risks.
What happens to the AAV9 viral capsid after infusion?
Intact rAAV particles in endosomes undergo a series of pH-dependent structural changes necessary for transduction and traffic through the cytosol via the cytoskeletal network. After endosomal escape, rAAV enters the nucleus through the nuclear pore complex, where it undergoes capsid uncoating to release the genome. Following uncoating, a small fraction of viral peptides is targeted to the lysosome and degraded. (Wang D et al. 2019; Siddhartha Mukherjee, 2022)
Because AAV and associated parvoviruses naturally infect mammals, it is important that patients be tested for pre-existing neutralizing antibodies (NAbs), which could block AAV-mediated gene transfer and interfere with successful transduction. Global estimates of the general population suggest 70% of adults have no or low pre-existing AAV9 NAbs, but rates vary based on geography, demographics, and age (Verma, S., et al., Hum Gene Ther, 2023;34(9-10):430-438.). In our RIDGETM Natural History study of 144 patients with PKP2-associated ARVC, 93% of patients show AAV9 neutralizing antibody (NAb) titers ≤1:40, meeting eligibility criteria for TN-401 gene therapy.
Is AAV9 transmissible to household contacts/family members?
AAV vectors, including AAV9, are generally considered to have a low risk of viral shedding and transmission due to their non-pathogenic nature and inability to replicate in human cells. Therefore, they do not pose a risk of transmission to others. (Suckau et al., 2009). However, it is always important to exercise caution and follow appropriate safety measures when working with viral vectors to minimize any potential risks.
In general, most health care facilities recommend universal/standard precautions with patient material between 14 and 30 days after administration for both health care staff and direct family members, dependent on viral shedding analysis. Additionally, instructions are provided to family members and caregivers to practice good hand hygiene for a minimum of 2 weeks after the injection. This requires washing hands with soap regularly and using appropriate protective gloves if coming into direct contact with patients’ bodily fluids and waste. (Ghosh S et al., 2020.)
What are the most common side effects associated with AAV-based gene therapy and how often do they occur?
There are two types of immune responses: innate and adaptive. Most adverse events in gene therapy studies have been linked to adaptive immune responses. Adaptative/Cellular Immune Response (Hepatotoxicity) happens at week 1-12 after the vector administration.
Immune-mediated liver toxicities can be encountered when an AAV vector is administered systemically. The presentation of the symptoms is normally: transaminase elevations (ALT most pronounced), decline in transgene-protein expression, IFN- ELISPOT to AAV capsid peptides. It occurs 1 week post vector administration, with a second peak (higher) at 1 month, concurrent with steroid wean or discontinuation. In the Zolgensma clinical trial participants, the vast majority (90%) had elevation in AST and ALT, but importantly, none had elevations of bilirubin more than 2x ULN, though all these recipients were on prophylactic steroids. Notably, only a small minority had very high elevations in liver enzymes.
What is the risk of myocarditis with AAV gene therapy?
The risk of myocarditis with AAV gene therapy appears to be very low but is a noted concern, particularly in specific patient populations, such as those receiving treatment for conditions like Duchenne muscular dystrophy (DMD) in which cases of myocarditis have been reported during clinical trials, including the death of one patient. Based on the investigation by an external data monitoring committee, all cases of myocarditis that occurred appeared to be associated with a specific pattern of genetic mutation in the DMD gene. Enrollment criteria was subsequently adjusted and no further incidents have been reported to date. (C.G. Bonnemann, 2023)
Although the etiology of AAV vector-induced myocarditis is not yet fully understood it seems feasible that the inflammatory milieu of the damaged muscle tissues in DMD patients favors induction of an immune response to the AAV vectors’ transgene product or alternatively that the added inflammatory reaction induced by the AAV vectors promotes auto-reactive T cell responses to muscle cells. (Ertl, 2022)
Continuous monitoring and safety evaluations are crucial in ongoing trials to assess the incidence and severity of this potential side effect.
What is the risk of mutagenesis with AAV gene therapy?
To date, there have been no reports of AAV-induced tumor formations in humans.
AAVs are generally non-pathogenic and do not integrate into the host genome in a manner that leads to significant mutagenesis. However, there are concerns about potential insertional mutagenesis, where the therapeutic gene could inadvertently disrupt important genes or regulatory elements in the host DNA. Ongoing research is focused on better understanding these risks and enhancing the safety profiles of AAV vectors.
Recombinant AAV integrations leading to hepatocellular carcinoma have been reported in mice. In a large study of 695 mice evaluating serotypes 1, 2, 5, 7, 8, and 9, only one liver tumor was observed resulting in an incidence of 0.14% (Bell P, Wang L, Lebherz C, et al. 2005). In total, AAV2 posed the greatest risk of insertional mutagenesis (Bell et al. 2005).
TN-401 General Aspects
Basic explanation of gene therapy construct formation/how is this manufactured?
Gene therapy constructs are created by encapsulating a therapeutic gene within a capsid (vector), which is typically a virus (like AAV). Within the capsid, certain regulatory elements, including organ-targeting promoters, can deliver the therapeutic gene (transgene) into target cells. In TN-401, a cardiac-specific promoter facilitates expression of the transgene in cardiomyocytes and limits the off-target expression of the transgene.
The manufacturing process involves several steps:
- Gene Cloning: The therapeutic gene is inserted into a plasmid, which serves as a template. The plasmid contains the AAV “inverted terminal repeat” (ITR) sequences and a cardiac-specific promoter, in this order: 5’-ITR —promoter-human PKP2– ITR-3’. The ITR sequences are derived from AAV and are necessary “messages” for the DNA construct to be subsequently packaged into an AAV capsid during cell culture production. Also, the ITR sequences are the only sequences of viral origin remaining in the construct.
- Vector Production: The plasmid is transfected into producer cell lines, which then produce the viral particles containing the therapeutic gene.
- Purification: The viral particles are isolated and purified to remove any impurities.
- Quality Control: The final product undergoes rigorous testing to ensure safety, efficacy, and consistency.
Why did Tenaya Therapeutics select AAV9 to treat PKP2-associated ARVC?
AAV9 has the most well-established clinical validation as compared to any other capsid and has demonstrated safety and durability in thousands of patients (Novartis). AAV9 is also the only serotype to date that has demonstrated efficient transduction in cardiomyocytes (Rocket Pharma). In most tissues, AAV9 appears to achieve cell transduction with efficiency superior to other AAV serotypes. Viral vectors based on AAV9 have also proven to be more efficient for murine, NHP, and porcine cardiac muscle transduction (Issa SS et al. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells March 2023; 12(5): 785).
A recent study by Cheng et al. compared cardiomyocyte transduction and transgene expression between three AAV serotypes: AAV9, AAVrh.10 and AAVrh.74. In mice, all three serotypes show similar transduction to the heart as measured by viral genome DNA load, however, AAV9 generates higher expression level of cardiomyocyte-specific RNA reporter transcripts than the other two serotypes manufactured in parallel on the same platform. Cheng et al. also measured heart transduction and cardiomyocyte transgene expression of all three serotypes in Cynomolgus monkeys and observed similar trends as those achieved in mice, namely that AAV9 mediates the highest level of cardiomyocyte gene expression. Although all three capsids can similarly transduce the heart overall, AAV9 specifically transduces and is expressed in cardiomyocytes more efficiently than AAVrh.10 or AAVrh.74 at the same dose level. A significant advantage of enhanced expression with AAV9 is that near-maximal doses are considerably lower than dose levels used in other gene therapy programs, with the potential for fewer dose-related adverse events (Cheng et al. ASGCT 2024).
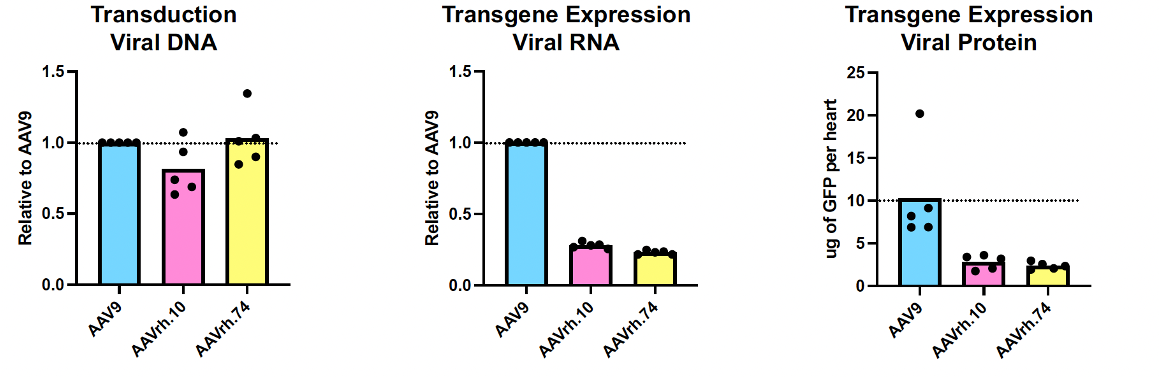
Why don’t you administer TN-401 through an intra-coronary route vs. intravenous (IV)?
Preclinical treatment in non-human primates was performed to determine the optimal route of administration for Tenaya’s TN-201 gene therapy program designed to treat MYBPC3-associated hypertrophic cardiomyopathy. IV dosing performed comparably to intra-coronary delivery (RCSI) in non-human primates based on transduction analyses for TN-201. The results showed that RCSI demonstrates similar cardiac expression of TN-201 as IV; however, it is associated with a greater risk of adverse events. Since our TN-401 gene therapy program utilizes the same capsid as our TN-201 program, we applied our learnings from our TN-201 preclinical results and determined it would be appropriate to administer TN-401 via IV.
Does TN-401 alter the patient’s genome (integration)?
AAV9 is generally considered non-integrative, making it a safer option for gene delivery in therapies aimed at non-dividing or slowly dividing cells. TN-401 AAV9-based gene therapy has a very low probability of genomic integration.
AAV vectors, including AAV9, primarily exist as episomes, which are separate from the host’s chromosomal DNA. This reduces the risk of insertional mutagenesis.
Are patients able to be re-dosed with TN-401?
Currently, a person can receive an AAV-based gene therapy only once. The viral makeup of the gene therapy construct primes the immune system to potentially attack the viral components the next time it sees the same or similar (other AAV serotypes) therapy. Redosing of AAV gene therapies is an ongoing area of research.
Will patients be able to stop their medications and avoid cardiac interventions after TN-401?
While gene therapy for inherited heart conditions like PKP2-associated ARVC is still under investigation, one of the potential benefits is to enable patients to stop or decrease other medications and devices currently used by patients to treat ARVC.
Does the patient continue to make dysfunctional protein alongside WT protein after TN-401 has been administered? Can dysfunctional protein compete with TN-401-derived WT protein within the sarcomere?
It is unclear how much mutant protein will be produced after TN-401 administration. The majority of PKP2 mutations are truncation mutations that are subject to nonsense-mediated mRNA decay, by which the mutated mRNA is degraded (Hug et al., 2016; Mura et al., 2013; Rasmussen et al., 2014). Therefore, if any trace amount of mutant protein is made, it is unlikely to compete with the TN-401-derived wild-type PKP2 protein.
Does it matter which PKP2 mutation an individual has in order for TN-401 to benefit?
Patients with confirmed pathogenic or likely pathogenic PKP2 mutations (loss-of-function variants) are eligible for TN-401 therapy. Mutations classified as truncating mutations include frameshift, nonsense, splicing, and large rearrangements. Mutations in PKP2, the gene encoding plakophilin-2 protein, are the most common cause of familial ARVC, an autosomal dominant condition with incomplete penetrance. In the majority of cases, the disease is caused by loss-of-function mutations in the PKP2 gene, which lead to a reduction in both mRNA and protein levels in ARVC hearts (Akdis, 2016; Asimaki, 2009; Chen et al., 2014; Kohela, 2021; Rasmussen, 2014). TN-401 is to replace the missing amount of wild-type PKP2 protein that is needed to power normal heart function.
How long does it take for the gene therapy to demonstrate benefit?
It is not yet known how long it will take for transduction, transgene expression, protein expression, or benefit to take place in humans. Based on the experience of other sponsors, we know that gene therapy can take several weeks to months to show efficacy (as opposed to the near-immediate effects possible with other therapeutic modalities), and that relatively low levels of protein expression appear to have growing benefit over time, and that results can be durable, lasting for several years.
Available preclinical data on TN-401’s efficacy in knock-out murine models of disease were published in Nature Communications.
How long will the effect of TN-401 last?
TN-401 has the potential to be durable. Cardiomyocytes are considered the most compatible for use of AAV gene therapy, as cardiomyocyte turnover is negligible in adults.
Long-term follow-up data for the approved AAV9-based gene therapy, Zolgensma, indicate durability greater than 15 years.
Latest data from two Long-Term Follow-Up (LTFU) studies, LT-001 and LT-002, show the continued efficacy and durability of Zolgensma across a range of patient populations, with an overall benefit-risk profile that remains favorable. (Mendell J. et al,2021). Highlighting the remarkable durability of Zolgensma, data from LT-001, an ongoing 15-year LTFU study of patients who completed the Phase 1 START study, showed that up to 7.5 years post-dosing, children who were treated after presenting symptoms of SMA maintained all previously achieved motor milestones.
The KO mouse model is homozygous and doesn’t accurately represent the heterozygous humans you will be studying in the RIDGETM-1 clinical trial. Will study participants see similar degrees of efficacy?
RIDGE TM-1 will investigate adults with heterozygous truncating mutations. In the cardiac knock-out mouse model, which presents the most severe ARVC disease course, TN-401 demonstrated improvements in LVEF%, reduction in RV enlargement and arrhythmia scores, most importantly, a significant survival benefit. TN-401 efficacy studies conducted in the cKO mouse model revealed a closely regulated dose-function relationship that supported the mechanism of action of the gene replacement approach.
Supported by this dose-functional relationship, restoration of the missing WT protein in heterozygous human patients is expected to be efficacious. However, real-world data show that penetrance of PKP2 mutations is influenced by age, gender, and environmental factors that result in heterogeneous disease presentations.
Will TN-401 work if the heart has too much fibrosis?
To demonstrate how TN-401 can slow down fibrosis development and disease progression, we used a cardiac-specific knock-out, Pkp2-cKO, mouse model that recapitulates human ARVC clinical phenotypes. We demonstrated the TN-401 effect in two disease models: before the cardiomyopathy phenotype development (preventive model) and in overt cardiomyopathy (treatment model).
In the preventive model, TN-401 significantly reduced fibrosis development and collagen deposition in both ventricles (Figure below).
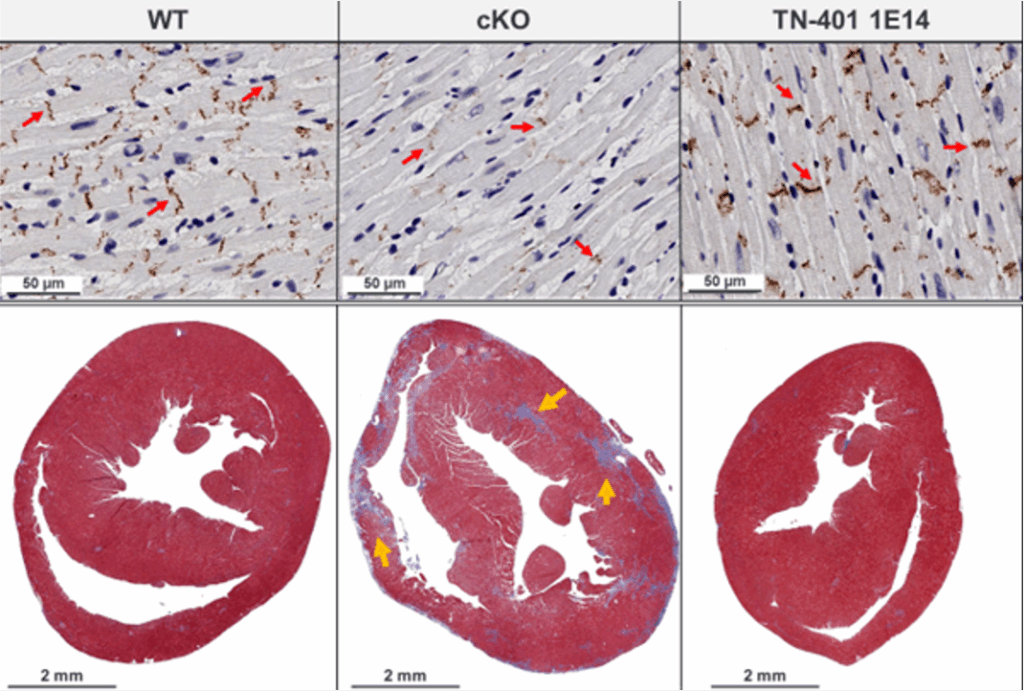
In the overt disease model (therapeutic), a quantitative gene expression analysis showed that TN-401 treatment reduced mRNA expression of heart failure markers and fibrosis genes (Timp1, Col1a1, and Col3a1); however, the reduction of existing fibrosis was not shown.
Based on these studies, we believe that if overt structural changes have occurred, gene therapy is unlikely to reverse those effects, for example, in removing fibrosis or in replenishing more cardiomyocytes (Wu et al., 2024).
RIDGE<sup>TM</sup>-1 is focused on patients with preserved systolic function, where most of the cardiomyocytes are functional. As part of the clinical trial eligibility criteria, participants who are most likely to respond to gene therapy will be required to have an LVEF >50%.
How do you measure the transgene expression to determine if PKP2 expression is restored?
Early efficacy and durability of transduction will be assessed through vector genome DNA and transgene mRNA, and protein expression levels from RV septal biopsy samples. A right heart catheterization and endomyocardial biopsy will be performed at baseline, post-dose, and Week 52. The first post-dose biopsy will be used to assess early efficacy, and the biopsy at Week 52 will be used to assess the durability of the TN-401 effect. These results will inform future dose selection.
In the context of gene therapy, VCN (Vector Copy Number) or vector genome refers to the number of therapeutic gene-carrying vectors that have been successfully introduced into a patient’s target cells.
Vector Genome: The vector genome is the genetic material within the vector that contains the therapeutic gene. This genetic material is what gets delivered into the target cells to carry out the intended gene therapy. In some cases, the vector genome may also include regulatory elements to control the expression of the therapeutic gene.
Vector Copy Number (VCN): VCN specifically refers to the number of vector genomes that have been transferred into the host’s cells. The higher the VCN, the more copies of the therapeutic gene, potentially resulting in a more robust and sustained therapeutic effect.
The VCN is a crucial parameter in evaluating the efficacy and safety of a gene therapy treatment. A higher VCN generally indicates a more successful gene delivery, which can be important for achieving the desired therapeutic outcome.
Monitoring and measuring VCN is part of the overall assessment of a gene therapy’s effectiveness and safety in clinical trials and patient treatments.
Total PKP2 protein levels will be quantified by mass spectrometry in the patients’ baseline, 8-week, and 52-week RV septal wall biopsies to monitor for increased PKP2 expression. Patient PKP2 levels will be compared to levels in normal hearts to determine the level of restoration. Therefore, it is critical to perform biopsies at protocol-directed windows.
Is there a way to remove the transgene after it has been administered?
No, TN-401 gene therapy is designed to directly restore the PKP2 protein content in the cardiomyocyte, whose deficit is the biological basis of this genetic cardiomyopathy, which would be irreversible.
TN-401 Safety
How often has TMA occurred with gene therapy?
TMA from AAV gene therapies is rare overall, especially with widely used vectors like AAV9. However, certain applications—especially high-dose or less common capsid therapies—present significantly higher risks in small cohorts. To enhance the safety and effectiveness of AAV gene therapies, it is crucial to implement a comprehensive approach addressing the complexities associated with treatment-induced TMA. Monitoring for TMA and eligibility criteria adjustment have become critical components of trial design and product safety management.
Among >1,400 patients treated with Onasemnogene abeparvovec (Zolgensma®, AAV9 for SMA), 37 cases of TMA were reported via FDA’s adverse event data → ~0.9% prevalence (Genevieve A. Laforet, 2025 Thrombotic Microangiopathy Associated with Systemic Adeno-Associated Virus Gene Transfer: Review of Reported Cases | Human Gene Therapy)
- In the RESTORE registry for Zolgensma (n = 167): 1 case (0.6%) was documented
- In a global managed access program for Zolgensma (n = 102 adults): 1 case (0.9%).
- In Danon disease, 1 of 6 patients who was treated with high-dose AAV9 of 1.1×1014 genome copies per kilogram, has developed TMA (B. Greenberg et ail, 2024 DOI: 10.1056/NEJMoa2412392)
More recently, complement activation has been reported in adult subjects with Fabry’s disease receiving one single dose of 4D-310 (AAV2 capsid variant) in combination with prophylactic oral corticosteroids, thus suggesting that these phenomena are likely not AAV9-specific (Salabarria SM et al, 2024, Thrombotic microangiopathy following systemic AAV administration is dependent on anti-capsid antibodies – PubMed). Another case is with LK03 capsid for methylmalonic acidemia (MMA): TMA occurred in 2 patients in the younger age group, prompting protocol revisions ((Genevieve A. Laforet, 2025 Thrombotic Microangiopathy Associated with Systemic Adeno-Associated Virus Gene Transfer: Review of Reported Cases | Human Gene Therapy)
In most TMA cases, the data suggest that the symptoms start 6-12 days after gene therapy administration, and that patients have been treated successfully with a complement inhibitor (eculizumab), an increase in steroid doses, plasmapheresis, hemodialysis, and platelet transfusions. Hospitalization with daily safety laboratories from Day 1 to Day 7 is included as part of the RIDGE TM-1 study protocol to enable intensive monitoring and early treatment for hepatoxicity and TMA. As another risk mitigation strategy, AAV-mediated gene therapy protocols now include the addition of a complement-targeted prophylaxis agent like eculizumab, a C5 inhibitor, to manage TMA if it develops (Guangping Gao, 2024 https://doi.org/10.1038/s41392-024-01780-w, Ertl H, 2022, FDA Advisory Committee 2021).
What is the risk of overexpressing PKP2 protein following TN-401 therapy?
The highest overexpression level of PKP2 in either cKO or WT mice is about 4-fold higher than the WT level when evaluated in both efficacy and pilot safety studies. PKP2 protein expression occurred at doses as low as 1E13 vg/kg. Based on the results obtained from both GLP safety and toxicology studies, the no observed-adverse-effect levels (NOAELs) of TN-401 were manyfold higher than the proposed first-in-human doses.
How much PKP2 protein levels could be enough for normal heart function?
This will ultimately be determined in our clinical trials. We have reason to believe that a relatively modest increase in protein levels may infer clinical benefit and have designed our Phase 1b RIDGETM-1 clinical trial to capture numerous exploratory endpoints to better understand the relationship between changes in protein levels and potential changes in disease burden and progression.
Our preclinical studies (Figure 5, 7, 8, and Suppl. Figure 5, Wu et al., 2024) demonstrated the dose-function relationship of the gene therapy, showing the level of PKP2 expression correlates with the extent of the phenotype correction. In these studies, 3E13 vg/kg achieved near maximal efficacy, with nearly all cardiomyocytes transduced.
Do we anticipate seeing a drop in ejection fraction in human subjects following the infusion of TN-401?
A drop in EF is not expected; Per protocol, patients will be rigorously monitored for changes in EF using echocardiography (full and focused Echoes) up to 8 times post-dose.
Is transgene expressed in other off-target tissues like the liver?
Based on our preclinical toxicology studies, we did not observe adverse liver toxicity. We would expect similar off-target activity to that observed in other AAV9 clinical studies. Based on the experience of other sponsors, we know that dose-related expression in the liver happens and is generally transient, monitorable, and treatable. The RIDGETM-1 study is designed to monitor patients closely for any potential adverse events.
Are patients at increased risk if they have had myocarditis following the COVID-19 vaccine?
In general, patients who have experienced myocarditis following a COVID-19 vaccination may face increased risks of myocarditis, an inflammatory condition affecting the heart. Immune or inflammatory triggers—such as viral vectors—could potentially exacerbate the condition.
In our preclinical models, no cases of myocarditis were observed. We do not expect that PKP2-associated ARVC patients develop myocarditis in response to PKP2 protein, as the patients are heterozygous, and they likely have immune tolerance to this protein.
Nevertheless, in our Phase 1b study, our safety monitor for inflammation is very rigorous, including monitoring for inflammatory proteins, cardiac biomarkers, and changes in heart function based on echo.
How long does the patient need to take immunosuppression?
The duration of immunosuppression during gene therapy trials can vary depending on the specific therapy, the vector used (such as AAV9), and the patient’s immune response. Generally, immunosuppressive regimens may last from a few weeks to several months after gene therapy administration to minimize immune reactions to the viral vector or modified cells. The goal is to ensure the body accepts the therapy without rejecting the treatment or causing inflammation. Monitoring and adjustments are made based on individual patient needs and responses.
In the RIDGETM-1 Phase 1b study, the length of the short-term immunosuppression will be determined by individual responses to TN-401. The immunosuppression regimen for RIDGETM-1 aims to diminish the immediate effects of the innate immune reaction and the subsequent adaptive T-cell responses to the AAV9 capsid. Of note, because PKP2-associated ARVC patients in this study are heterozygous and express PKP2 protein, they likely have immune tolerance to this protein, and so adaptive responses to the TN-401 transgene product are not expected.
Are the patients eligible for heart transplant after the AAV gene therapy?
There are no absolute contraindications for heart transplantation after AAV gene therapy; however, the patient’s immune response, therapy efficacy, and clinical condition are critical in determining eligibility. If the gene therapy successfully halts disease progression or improves heart function, it might delay or even prevent the need for a heart transplant. However, if the gene therapy’s effects are insufficient or short-lived, the patient may still require transplantation, and they are still eligible for a heart transplant.